LESSON 3
a. pH values. The pH scale extends from 0 to 14, with 7 representing
the pH of pure water at 25 degrees Celsius, or any perfectly neutral
solution. On the pH scale, numbers greater than 7 indicate the degree of
alkalinity. As you proceed up the scale to 14, a pH of 8 represents a
slightly alkaline solution and a pH of 14 represents the strongest alkaline
solution. Moving down the scale from 7 to 0, indicates the degree of
acidity. A pH of 6 indicates a slight acid solution and a pH of 0 indicates
the strongest acid.
(1) The relative strength of acids and alkalis changes ten times for
each unit change in pH. Compared with a solution of pH 6, a solution of pH
5 is ten times stronger, a solution of pH 4 is a hundred times stronger, and
a solution of pH 3 is a thousand times stronger than a pH 6. This is also
true of the alkaline scale 7 to 14.
(2) Acids and alkalis are considered to be strong chemicals and
therefore must be handled very carefully. Acids and alkalis are quite
capable of causing serious skin damage. When preparing solutions containing
these chemicals (compounds), always follow the CAUTION and WARNINGS listed
on the containers.
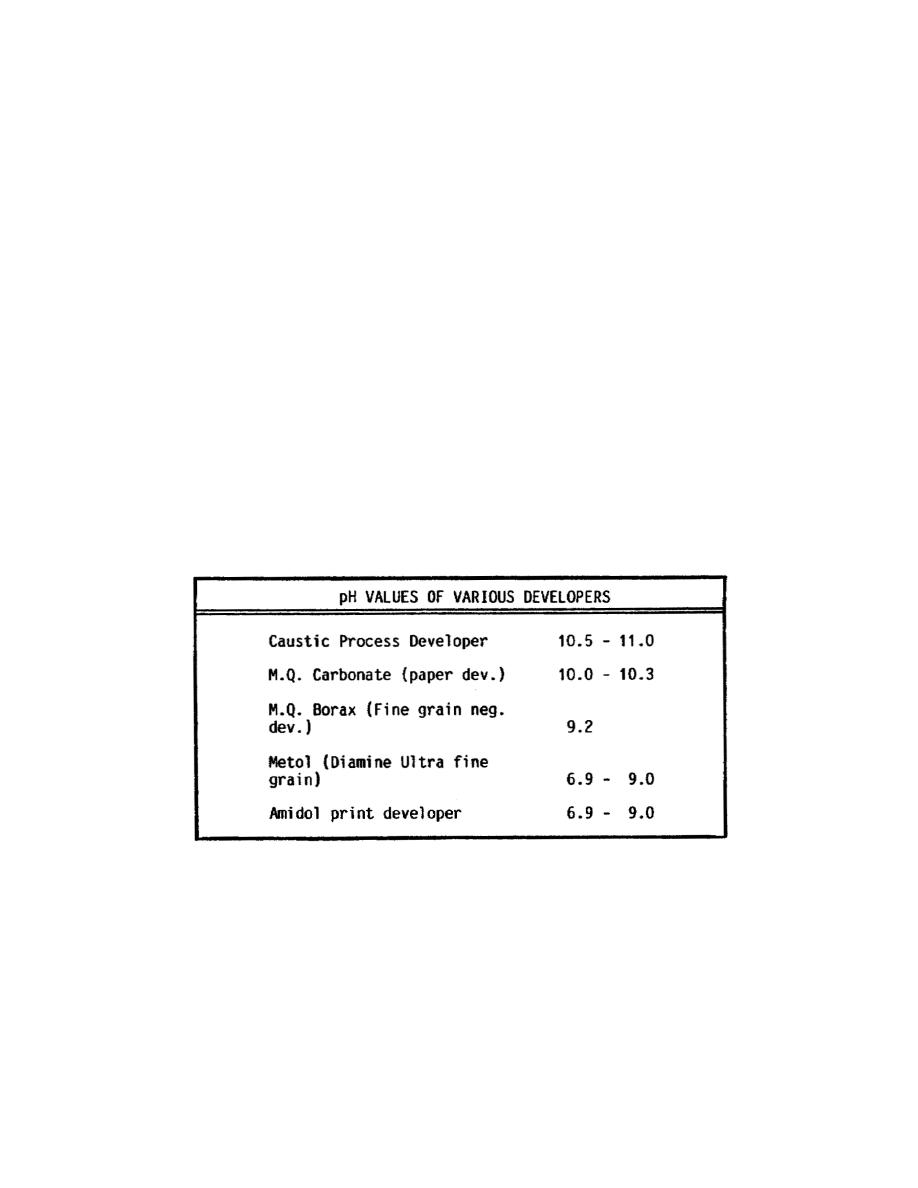
(3) Generally, a developing solution must be alkaline in order to
reduce silver halides efficiently. Refer to Figure 34.
Figure 34. pH values of various developers
b. All pH measurements must be obtained relative to a standard
reference solution. This means that every accurate pH measurement actually
requires two measurements; one for the
93



 Previous Page
Previous Page
